Gene Editing and Gene Therapy History and Regulations in a Nutshell: Dr. David Segal
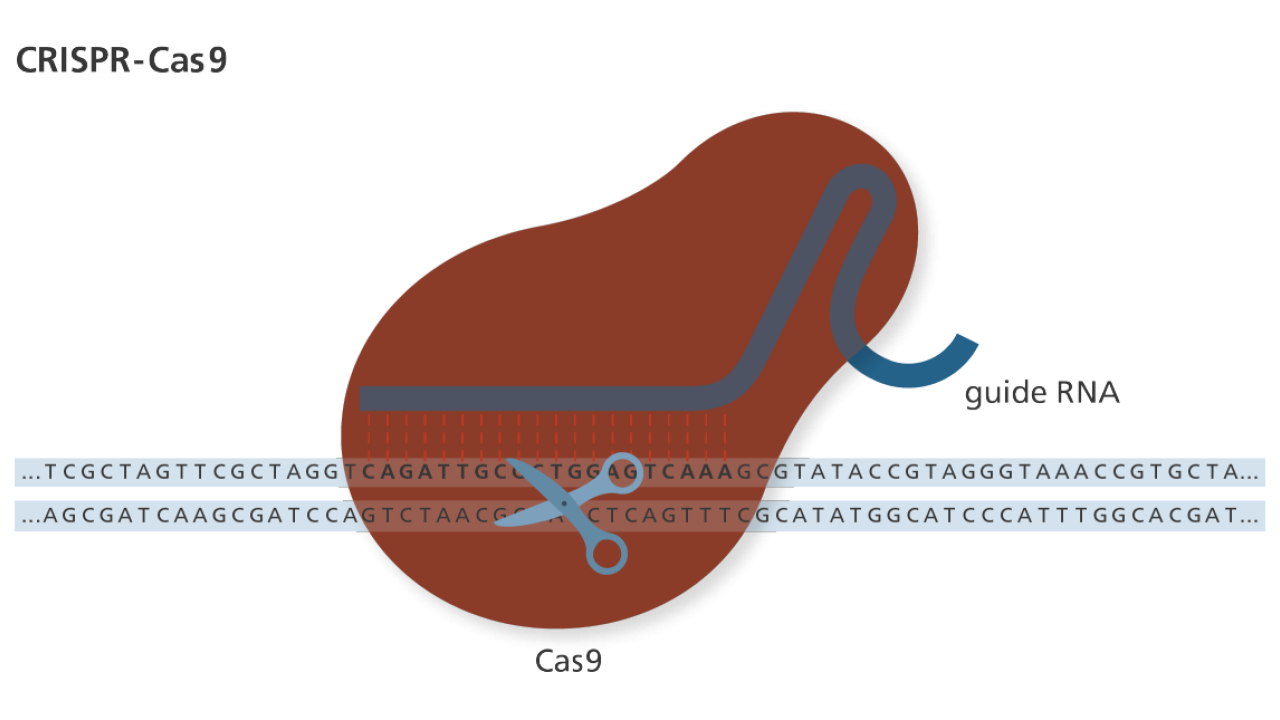
 Reports from China regarding Dr. He Jiankui’s human embryo edits using CRISPR-Cas9 have continued to catch the eye of the popular media months after the initial report. Some articles dig into whether Dr. He’s work was ethical, while others suggest that his intention was to improve their intelligence rather than making them genetically immune to HIV. I spoke with Dr. David Segal of UC Davis to get a better understanding on genetic editing history and the regulations we have in place to truly understand if CRISPR technology is as scary as is implied by the media. A transcript of our conversation follows, edited for clarity.
Reports from China regarding Dr. He Jiankui’s human embryo edits using CRISPR-Cas9 have continued to catch the eye of the popular media months after the initial report. Some articles dig into whether Dr. He’s work was ethical, while others suggest that his intention was to improve their intelligence rather than making them genetically immune to HIV. I spoke with Dr. David Segal of UC Davis to get a better understanding on genetic editing history and the regulations we have in place to truly understand if CRISPR technology is as scary as is implied by the media. A transcript of our conversation follows, edited for clarity.
Sydney Wyatt (SW): What is your experience with genetic editing technology and your place in the history of its development?
David Segal (DS): I was a graduate student with Dana Carol, who is now considered one of the elders in [the genetic editing community. Mario Capeechi, who would later go on to win the Nobel Price for his work in gene targeting, developed an approach that only worked in one in a million cells. Many people were trying to understand what the slow step was and Dana Carol thought that if we made a targeted double strand break (DSB) in chromosomal DNA, we could stimulate this targeting by hundreds of thousands of fold. Back then, there was no good way to make those targeted breaks, so we had to use a naturally occurring targetable nuclease. It set up this quest for targetable double strand cleavage reagents. In my postdoc, I worked with zinc finger proteins that eventually went on to make zinc finger nucleases (ZFNs).
Flashing forward, there are still ZFNs, and now TALENs and CRISPR. We’re still doing lots of work with these targetable technologies: gene editing where we cut the DNA, or epigenome editing where we just affect the epigenetic layer of information. We’re trying to use them as tools to study genetic and epigenetic processes as well as developing them as therapeutics.
Currently in my lab, we are trying to develop an epigenetic therapy for Angelman Syndrome. We work closely with Kyle Fink, who is trying to develop a target therapy for Huntington’s Disease. Between our labs, we’re interested in six or seven neurologic diseases that we think are treatable using this technology.
SW: Dr. He Jiankui, the researcher behind the “CRISPR babies” in China targeted CCR5 for deletion to provide immunity to HIV. This has been the target of other gene editing strategies in the past. What about CCR5 makes it so attractive?
DS: There’s a certain set of common targets, but CCR5 is probably one of the most logical ones. In terms of mutated genes, we’re trying to fix it so it will function normally. In the unusual case of HIV, this receptor CCR5 can be mutated and essentially the virus will just bounce off the surface of the cell. It’s not that we don’t need the CCR5 gene, but there seem to be redundant functions. We know this is the case because there is a naturally occurring CCR5 mutation that provide those people with HIV resistance but seem phenotypically normal. So instead of needing to fix the gene, we need to mutate it, which in some ways is simpler.
We think about gene therapy as introducing a new gene into a person, so a gene transfer is involved, whereas gene editing actually changes an endogenous gene.
CCR5 is a popular target because we can do somatic cell gene therapy for HIV. We can remove the target cell population, treat them in a cell culture dish in the lab in order to create a CCR5 mutation to make the cells immune to HIV, and reintroduce them to the patient. This an ex vivo gene therapy model. And diseases that are able to be treated in this manner are going to be the first to have gene therapy treatments developed. HIV, via CCR5, is in that group along with beta thalassemia and sickle cell anemia.
SW: You mentioned ex vivo gene therapy is a form of somatic cell gene therapy. What is somatic cell gene therapy and the opinions about this in the scientific field?
DS: We think about gene therapy as introducing a new gene into a person, so a gene transfer is involved, whereas gene editing actually changes an endogenous gene. There are two classes of gene editing or gene therapy: germline gene editing or gene therapy and somatic gene editing or gene therapy. Somatic cell gene therapy is more like modern medicine in that someone has a disease and you treat that person, similar to what drug therapy can do. We can also think about germline gene therapy in which we can treat either an embryo or affect the gonadal tissue on purpose or accident. This can actually change the gene of interest not only in the person that grows up, but all of their children after that.
I think that when science starts to push up against ethical boundaries, we have institutions in place to try to respect society’s concerns about the research being conducted.
Clearly this germline gene therapy represents a less well understood area with potentially long term consequences that would affect people that have not given consent to this but would bear the effects if something went wrong. It’s a complicated issue and talked about extensively in the field. I think most people would prefer to work with things that don’t have large controversies surrounding them in order to move gene editing and gene therapy technology forward. There is a strong sense that society is not ready for germline gene therapy, on top of the existing concerns about somatic cell therapies. Until there’s a good track record for it, germline gene therapy generally shouldn’t be pursued at this time.
SW: When the gene editing field was brand new, editing efficiency was very low — one in a million cells actually got the intended edit. I would also be concerned about off-target effects, or “incorrect” edits. Since CRISPR-Cas9 is the newest gene editing technology, what are the possible off-target effects and what are the potential consequences?
DS: There were concerns early on about off-target effects. Now there are many high fidelity Cas9’s that show lower off-target effects thanks to optimization efforts. We published a review on CRISPR specificity and found that off-target effects are relatively rare if you couple a very careful analysis of guide RNA choice with high fidelity nucleases, the odds of getting off-target events are dramatically reduced. But there are other problems that are greatly concerning to me that aren’t being talked about as much as off-target effects.
Every study that’s looked for these kinds of chromosomal rearrangements has found them, but we don’t usually look for them. It’s a big theme in the field: you only see what you look for.
First of all, making point mutations or small indels somewhere else in the genome other than your target site could cause problems. Only 2% of the genome is protein coding, so off-target events will likely be in a non-protein-coding region. In this case, there might not be a negative effect, but off-target events could lead to genetic rearrangements. Every study that’s looked for these kinds of chromosomal rearrangements has found them, but we don’t usually look for them. It’s a big theme in the field: you only see what you look for. And things we haven’t looked for are starting to come out now, such as big rearrangements in chromosomes, translocations, inversions, and deletions. If there’s an off-target event near the intended cut site, it’s likely that there will be one of these rearrangements. When you get into CRISPR-Cas9 editing in vivo and are affecting a lot of cells, the likelihood of this rare off-target rearrangement event is going to go way up.
Another problem that emerged just last year is that even a break at the correct target site can lead to unintended or unappreciated genetic repair. When checking for CRISPR-Cas9 activity, we perform a PCR reaction to look at the target site to determine if it’s been modified. But what if we look at a much wider area of 5-10 kilo bases? We find things that aren’t picked up by the small PCR such as large deletions of thousands of base pairs, non-contiguous repairs, or insertions. Other kinds of DNA can even insert into the space in a DSB, such as part of a donor construct.
A lot of gene editing development has been done in cell culture, often with cancer cell lines which already have messed up DNA repair that allows them to grow indefinitely. Recently, it’s been shown that primary cells from animals or people are a lot more sensitive to DSBs than these cancer cells are. For example, induced pluripotent stem cells (IPSCs) are very difficult to edit. When we introduce a DSB, there’s signaling in the cell that is necessary to trigger the repair pathway and all the events we want to happen. Using a nuclease to make those DSBs gives a very unusual signal. It will cut at the target site, the site hopefully gets perfectly repaired, and then it cuts again. And so on for hours to days at the exact same site. And many cells just apoptose, or die, because they continuously receive signals about DNA damage. We can inhibit the activity of p53, a component of the apoptotic pathway, but p53 perturbations are common in cancers. The cells that do survive without using p53 inhibition and are reintroduced to the patient might have a damaged DNA repair response that facilitated their survival. We might unknowingly select for cells that can potentially turn into cancer.
SW: Given all of these concerns, what are the funding regulations regarding gene therapy and gene editing? Are they fairly restrictive?
DS: Gene therapy efforts started in the 90s, so the United States Food and Drug Administration (FDA) has had a long time to think about what's worked, what hasn't worked, what are the questions they should be asking, what do you need to do to show that as therapy is safe? These questions have already been addressed by that community over decades. Now, the field and the FDA have some ideas about the toxicity of different factors, how to look for that toxicity, and how long you need to treat someone before they develop symptoms related to the therapy itself. Not all the question have been answered, yet there are some gene therapies that have actually been approved by the FDA. Not just approved to try them in people via clinical trials, but approved for marketing that a doctor now can order this gene therapy. They are considered to be safe and efficacious according to the FDA.
It’s absolutely essential that all the work go through IACUC and IRB to address the ethics and safety of this work.
In the area of gene editing, we're just getting to the stage now where we can start figuring out regulations for research purposes and human use purposes. With regards to research using animal models, all the safety and ethical concerns need to be addressed as you would for any other animal model experiment. If those animal models can contribute to learning about what effects a treatment might have on a disease in people, then we can see the lifesaving advantages of that research. Based on risk-benefit analyses, research using animal models may be performed only if there is going to be a very clear benefit from the harm or potential harm to the animal. And for some people, using animal models in research will never be ethical. This ethical dilemma is governed at every institution by an institutional animal care and use committee (IACUC). No animal work can be done without the approval of this committee. This committee is made up of people who are at an arm’s distance from the research. Usually there are members of the public on these committees to maintain transparency. And the government’s rules about what is and is not allowed are passed down to the researchers through this committee.
When it comes to actually trying to use these technologies in people, I don’t know if anyone, including the FDA, have really figured out what critical questions to ask. There is an IACUC counterpart for research involving humans, whether in a psychological survey or a clinical trial, and that’s the institutional review board (IRB). The field is trying to allow research to move forward to a point where we could use these technologies to treat people, and that means doing clinical trials. But we want to do it as safely as we can. It’s absolutely essential that all the work go through IACUC and IRB to address the ethics and safety of this work.
SW: These regulations seem fair and well thought out. As you said, some regulations have evolved over decades. Could you explain some of the ethical concerns and regulations specifically regarding germline gene therapy or gene editing?
DS: In animal models, I don’t think there are concerns that have reached a state of legislation, but the same rule applies: all work must be approved by IACUC. When it comes to human embryo research, that it a very highly charged issue on many levels, gene editing or not. In this country, and many countries, there are strong feelings about what can and cannot be done with human embryos, and we have to respect the wide range of opinions.
When it comes to human embryo research, that it a very highly charged issue on many levels, gene editing or not.
Some believe the potential benefits would tip the ethics in favor of using human embryos, not make people from the experiments, but to understand the limitations of the approach and what needs to be improved if this approach were to be used in people. Others would not accept this until there are other successes. If some of these ex vivo somatic cell gene editing approaches look successful and they really help people, not just change some gene expression but take a person from death’s door and allow them to live, I think people will start to feel like these approaches are a little bit more comfortable.
And our society is comfortable with some very dangerous things. Cars kill more people than almost anything else in our society; it’s the leading cause of death for people under the age of 20. And we’re all comfortable with cars because we see the benefits. To put this into perspective, if you went back in time to the horse-and-buggy era and introduced the cars of today as being really beneficial but that they will kill a lot of people, maybe the horse-and-buggy drivers will think “I don’t want to get these cars.” Or take smoking. There is no advantage to smoking, only disadvantages. Yet many people put this in perspective and say “I don’t feel sick today.” People’s attitudes towards things vary depending on what they see. It could be that some people will be swayed when they see successes and then there'll be other people for whom research on embryo will never be an option. That will never be okay.
SW: In 1975, there was a conference in Asilomar about developing regulations regarding recombinant DNA, and included scientists, lawyers, journalists, members of the public. At the time, there was concern that recombinant DNA could be used for human gene editing, and a moratorium was issued until the regulations could be hammered out. Interestingly, Dana Carol and Jennifer Doudna and others proposed a similar recommendations a few years ago. They were 1) discourage any research aimed at heritable genetic modification; 2) establish forums for information and education on the risks and benefits of using CRISPR to treat or cure human disease and to address ethical, social, and legal concerns; 3) support transparency to determine whether clinical applications are permissible; and 4) gather experts in genetics, law, and bioethics as well as the general scientific community and public to discuss these issues. What is your opinion on these recommendations and do they already exist?
DS: Except for the first one, the others have come to pass. There have been forums established to provide information, including this interview. There’s plenty of talk about these issues in the public press. There’s been multiple stories on NPR, usually when something bad happens. In fact, even Paul Knoepfler from our own UC Davis interviewed Jennifer Doudna; he’s very vocal about this subject and has even done TED talks. He’s just one guy. There are people like that from all over, representing many institutions. It is a rapidly evolving field and I think it's very hard to get the current state out, even to the scientists that are in it much less to the public that is receiving it very after the fact. By the time a grant we’ve written is funded, half of the proposed projects have either been done by someone else or the technology we proposed to use is obsolete. You feel like you’re always behind in this field; as soon as you start something you’re already behind.
One response to these recommendations was forming gene editing conferences, such as the International Summit on Human Genome Editing. The first conference was very big and even David Baltimore, who was at Asilomar, was there again. Dr. He actually presented his work at the second conference. On some level this was a chance for impassioned speeches, but the subject of whether there should be a ban on human gene editing research outright was certainly discussed. Some countries have done that; ours has not.
We need to have people in the field introducing these recommendations for debate. I personally feel that within the structures we have — IACUC and IRB — ethical research can be done in this area and we could learn a lot about life-threatening diseases. But it needs to be done in a transparent and ethical way that’s agreed upon after debate.
SW: NPR put out a really interesting article about gene editing in human embryos. Dr. Dieter Egli of Columbia University is using human embryos to edit one of the genes responsible for causing inherited blindness. He fertilizes the egg along with injecting all of the gene editing components and then allows it to develop one day. In the article, he states that he is solely doing this for research purposes. His hope is to eventually allow the embryos to develop longer in order to continue determine the actual effects of the gene edit. Given the tension surrounding human embryo editing, what’s your opinion on Dr. Egli’s work? Do you think these experiments would be considered a “research purpose” or “human use purpose” as you described earlier?
DS: I think that when science starts to push up against ethical boundaries, we have institutions in place to try to respect society’s concerns about the research being conducted. As I mentioned before, these concerns are addressed by IACUC and IRB when it comes to research involving animals and humans, respectively. And again, it is absolutely essential that all research involving animals or humans are submitted to these committees for approval.
If we don’t train the investigators, if we don’t go through the IRB, if we don’t follow the agreed-upon procedures, the investigator could lose their job. The NIH can stop funding the entire university. There are huge consequences.
Furthermore, all of the people performing research using human subjects need to be trained in the ethics of human research and how we need to avoid unethical behaviors based on past examples. These institutions function to maintain that decisions are being made in a transparent and rational and ethical way and that society should know that that is the process that’s keeping them safe. So there is an agreement that when we do research involving animals or humans, these ethical criteria have been met. If we don’t train the investigators, if we don’t go through the IRB, if we don’t follow the agreed-upon procedures, the investigator could lose their job. The NIH can stop funding the entire university. There are huge consequences.
And I would say that was the most egregious thing that scientist in China did. He didn't invent any new technologies. It's not like people have been trying to do what he did and they just couldn't figure out how to do it and he made some breakthrough discovery that allowed him to do it. It was nothing like that. He used technology that other people had shown to work and he did the same thing. The only thing he did different was that he went around all the safeguards that we have in place to maintain the society’s trust that scientists aren’t going rogue. Categorically, we cannot condone this work because it violates the systems that have been put in place.
SW: You’ve mentioned many good reasons to be hesitant for using nucleases for treatment. Yet there are biotechnology companies like Sangamo and CRISPR Therapeutics that are using this technology for gene therapy in clinical trials. What’s the safety of these treatments and what is the likelihood of these therapies working?
DS: The things that go into clinical trial, by government regulation, have to pass certain thresholds for safety and efficacy. We go to great lengths to demonstrate safety above all else, even efficacy, when we progress to clinical trials.
Sangamo was the first company to use ZFN to edit CCR5 in an ex vivo clinical trial. When they started this trial, no one had ever heard of CRISPR-Cas9. They expanded their portfolio to use ZFNs to make a targeted repair in the albumin gene in the liver in order to insert soluble factors to help patients with hemophilia or lysosomal storage diseases. This second approach is to deliver a transgene that is intended to be circulating around the body, such as factor-9 for hemophilia treatment. This produces copious amounts of the product in any tissue to help the patient, thereby avoiding trying to target the transgene to a specific organ. Certainly the animal models work well, and it’s early days still in this clinical trial.
I think CRISPR Therapeutics was also working on ex vivo gene therapy for beta thalassemia. Their clinical trial was halted by the FDA around the same time that the p53 story came out. The official word is that the FDA halted the trial because they wanted more information, but they didn’t say what information they wanted. They’ve recently become satisfied and the trial can move forward.
The things that go into clinical trial, by government regulation, have to pass certain thresholds for safety and efficacy. We go to great lengths to demonstrate safety above all else, even efficacy, when we progress to clinical trials.
I would say this is first time this technology has been tried in people, and to get to this point you have to demonstrate safety in animal models. To the best of the FDA’s ability to judge, it seems like these therapies should be safe for people. We won’t know for sure until the trials are completed, but this therapeutic approach isn’t going away. Whatever the outcome of these early attempts — fabulously successful, partially successful, or not successful at all — will set a baseline for us to build from.
Thank you to Dr. Segal for taking the time to answer our questions. For a detailed look into the history of gene editing regulations, check out “Don’t Fear the CRISPR” on UCD BioScope’s website.
Author: Sydney Wyatt is a PhD student at the University of California in Davis. For more content from the UC Davis science communication group "Science Says", follow us on twitter @SciSays.
Comments