
Dreaming of an Alternative Meat Future
An option for the uncomfortably carnivorous?

Many entrepreneurs are excited to potentially feed nearly half a million Americans yearly with a single animal cell – a possible technological solution to address mounting global protein demands. Several products have emerged to fulfill the rising need for additional protein to support a growing, hungry and increasingly upwardly-mobile population. This has resulted in more than $19 billion in investments for plant- and animal- based protein alternatives over the last decade (2010-2020, according to Pitchbook and Crunchbase).
Through innovative animal tissue culture technology, scientists have generated samples of animal cell-based meat (also called cultured meat or in vitro meat) as a potential alternative to traditional meat products. Cell-based meat is derived from biopsied animal cells, which are then grown using technology that allows scientists to mimic the structure of meat. This is different from meat substitutes derived from proteins and compounds extracted from plants and yeast, like products currently on the market from Beyond Meat® or Impossible™ Foods, or mycoprotein grown from fungal cells such as sold under the brand name Quorn®.

In contrast, cell-based meat is animal-derived and most like traditional meat in terms of composition at the cellular level. Although seemingly far-fetched, technology continues to advance and may someday allow products from animal cell-based meat to reach market affordably. However, high production costs remain a significant impediment that will require even more scientific advancement. While we may be a ways off from seeing these products reliably stocked on grocery shelves, we are on the technological cusp of being able to turn a strange science into a commercial reality… even if only the wealthy can afford the high-end cell-based meat products currently in the pipeline. Cell-based caviar, anyone?

Early days, humble beginnings
The advent of cell-based meat was predicted as early as 1930. Looking a century ahead, British writer and conservative politician Frederick Edwin Smith envisioned that by 2030 “from one ‘parent’ steak of choice tenderness, it will be possible to grow as large and as juicy a steak as can be desired.” Around the same time, Winston Churchill described a future in which “we shall escape the absurdity of growing a whole chicken in order to eat the breast or wing by growing these parts separately under a suitable medium.”
In the 1950s, Dutch innovator Willem Van Eelen went on to theorize that tissue culture could be used to generate cell-based meat and managed to secure a patent in 1999 with the emergence of in vitro stem cell cultures. Soon after, muscle biopsies were harvested from frogs and goldfish to explore cultured animal muscle protein for use on long term space flights or the habituation of space stations. To test for edibility with a panel of judges, the cultured meat was dipped in olive oil with spices, covered in breadcrumbs and fried. The verdict? Acceptable.
Fast forward to 2013, and the world’s first in vitro meat-based burger was produced in a lab using cells harvested from a cow’s shoulder over three months and costing more than $330,000 to grow. Assessed by a sensory panel in London, the five-ounce patty “almost” tasted like a conventional one, though lacked juiciness. As proof-of-concept, this was a step towards bringing cell-based meat to the public, but bringing a product to feasible market reality is still hampered by technological limitations and industry scale-up.
The first cell of many
Animal cell-based meat, also known as in vitro, cultured, or lab-grown meat, is produced through the culturing of cells outside the animal from which they are derived. To begin, adult stem cells are biopsied from an animal of choice (think cow, chicken or even fish) and transferred to a medium containing important growth factors to promote cell differentiation into mature cells. Typically, the cells used in animal cell-based meat production are classified as adult stem cells (different in origin from embryonic stem cells) that make identical copies of themselves over a long period of time, which is essential for generating a meat product. The specific stem cells targeted depends on the manufacturer, but generally includes muscle progenitor cells such as myoblasts or myosatellite cells. The combination of these two cell types is key: myoblasts make muscle cells that fuse to form muscle fibers, and myosatellite cells expand existing muscle fibers by adding nuclei. Together, they can create muscle fibers, but they need a little support first.
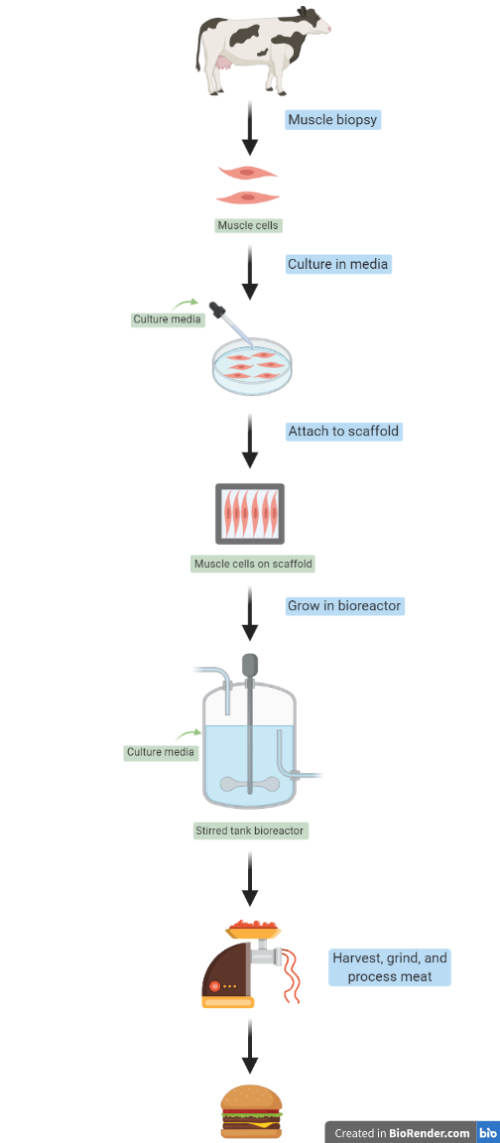
Muscle cells are anchorage-dependent, meaning they need to grip onto a material in order to divide and function properly. Inside an animal they would anchor onto other tissue, but outside the animal a scaffold is needed to replicate the cells’ native environment. A tissue-like stiff scaffold that mimics a real muscle’s environment with a large surface area for attachment and growth would facilitate optimal cell development, but a flexible scaffold would allow for beneficial spontaneous muscle cell contraction. Once the cells are supported on an appropriate scaffold, they then need the proper sources of energy and nutrients to aid growth. Of course, muscle cells alone are not enough to make a tasty meat alternative, so a combination of fat cells and other connective tissues must also be included.
The growth process occurs within a bioreactor, which is a large (25,000 – 1 million liter) environmentally controlled tank where a biological reaction can take place, similar to fermentation tanks seen at breweries or closed cheese making vats. They create an environment that supports a biological system and can potentially allow for high product yields, like those needed in harvesting animal muscle tissue. Yet commercial production is still to be tested publicly at scale within an industrial bioreactor.
Are we there yet?
Without fully developed technology, current production estimates and models must generalize from ideal scenarios and base-level assumptions. Challenges still exist with infrastructure development, system optimization, and costly inputs. The specialized bioreactors required are not yet in operation, and scaffolding techniques to generate different edible animal muscle products are still being theorized. Scaffolding would be used to produce ground and boneless meats with a soft consistency, but not highly structured meats like steaks. Additionally, initial idealized models did not take cell replication limits into account and made generous estimates of production capability, claiming in 2010 that 50,000 metric tons of meat could be produced from only ten stem cells grown over two months.
Animal cell-based meat is facing its greatest challenge economically. Current research focuses on making large-scale production more cost effective, and overcoming the limitations of expensive cell media and inefficient cellular metabolism. The culture medium supplying the cells with all the necessary nutrients such as vitamins, hormones, growth factors and proteins is one of the most expensive components of production but is vital for well-fed muscle cells. Estimates claim that 55-95% of production costs come from the growth medium alone, which will have to drastically reduce to compete with conventional meat production. Continued cell line development could also improve the metabolic efficiency of animal cells to be more competitive with the cost of farmed meat. Yet a recent techno-economic assessment of 2019 production costs estimated that replacing just 1% of traditional US beef production each year (121,000,000 kg) would cost almost $53 trillion to produce annually and would require more than 5,000 continuously operating 25kL bioreactors (the current size limit due to pressure constraints for mammalian cells). That works out to $148,664 for only 12 ounces of cell-based meat, just to break even! Innovation to bring down production costs will be critical to the success of the industry, as shown by the newly developed techno-economic cost calculator, a tool now available to assist future product planning and investment.
What's at "steak?"
The cost may be high right now, but cell-based meat also offers attractive possibilities, though plenty of unknowns. One compelling draw of cell-based meat is its potential to be more sustainable than farmed meat, where sustainable is defined as a product’s ability to meet the needs of the present without compromising the ability of future generations to meet their own needs. To evaluate this, we need a thorough life cycle assessment, which would systematically look at all the potential environmental impacts of cell-based meat products throughout the entire “life cycle” from production, to distribution, use, and disposal. In the absence of a full life cycle assessment, various studies have simplified the analysis in an attempt to generalize possible outcomes.
Techno-economic assessments to date rely on overly simplistic modeling, and older anticipatory life cycle assessments used inputs to calculate their analyses that never became a reality. Outstanding questions remain about both economic and environmental costs for variables from inputs to production (vitamins, minerals, nitrogen source, carbon source, phosphate source, sulfur source), transportation and downstream processing. Without a complete understanding of the input costs, greenhouse gas impacts and market share benefits of the final product, it is impossible to get a clear picture of the actual sustainability of cell-based meat. In the absence of this, publicly circulated claims about sustainability are so far relying on unverified private projections.
Socially, animal cell-based meat may attract consumers who enjoy meat but struggle ethically with animal treatment as a byproduct of carnivorous consumption. While there are several potential solutions to this moral quandary such as only purchasing meat that was slaughtered humanely, animal death is still unavoidable. Cell-based meat has the potential to offer a more humane product, but there is the drawback that the culture medium in which muscle cells grow commonly uses fetal bovine serum (FBS) derived from calves, although non-FBS alternatives are emerging. Currently, FBS is highly nutritious and effective at growing tissue but is still sourced from animals, which means that the final product fails to align with the goal of reduced animal impact. Therefore, cell-based meat products could provide uncomfortably carnivorous consumers with a desirable alternative, but the production methods will need to continue advancing beyond their current state.
Another proposed advantage of animal cell-based meat is that it could be carefully tailored for consumers’ optimal nutrition, while still being flavorful. Unlike conventional meat, cell-based meat’s nutritional profile has the potential for improvement during production without the genetic or environmental manipulation required in living animals. However, nutrition studies are far behind even feasibility studies for determining the health effects of cell-based meat’s reduced protein complexity, so rigorous research is still needed in this area.
Any negative effects of consuming animal cell-based meat are largely speculative at present. These products hold strong potential to carry less contamination and foodborne illnesses due to their sterile growth environment, but cell-based meat still needs to be processed in a system comparable to conventional meat, which could introduce similar risks. Cell-based meat production techniques could also hold a completely different risk profile, so ongoing attention needs to be paid to the food safety of future culture media additives.
The upshot is that there are a lot of unknowns with this new and evolving technology. Once companies are ready to start selling their cell-based meat products in stores, we’ll know much more about the sustainability, ethics, nutritional profile, and processing risks.
A fad, or the future?
One of the arguments against animal cell-based meat is its “yuck” factor. Nicknames like “Franken-meat” and “shmeat” have already emerged, suggesting issues with social acceptance. It is possible the public could object to cell-based meat for the same reasons genetically modified organisms (GMOs) prove controversial: they are perceived as “unnatural.” The definition of what constitutes a “natural” food is impossible to pinpoint, as humans have been altering their native foods for millennia. However, many consumers perceive “natural” to simply mean “not produced by humans,” and therefore cell-based meat would certainly face challenges with consumer perception due to its lab-grown origins. Conversely, the alleged “unnaturalness” of cell-based meat could be what makes it attractive to other consumers. On reflection, ethical scholars propose that it could provide something “superior to what nature offers – humans can live out their natural propensity to eat meat while also sparing animals from the horrors of that propensity.” Here in California, Science Says informally asked shoppers at the Davis farmer’s market about their willingness to try lab-grown (cell-based) meat and found that the majority of respondents (13 out of 17) were in favor of at least giving it a taste.
The color and appearance of cell-based meat also still needs work to match that of conventional meat, if the product aims to be a replacement. The commercial result of culturing animal cells is not identical to meat from an animal because animals possess inherently more complicated biological systems. The traditional meat we currently consume is generally composed of myriad cell types (muscle cells, ligament cells, nerve cells, fat cells, etc.), and producing an exact replica of this combination is beyond the realm of existing technology. Instead, cell-based meat could perhaps find a high-end niche market as a totally novel product.
Coming to a store near you, maybe!
Technological and commercial interests continue to push for animal cell-based meat to make the leap to market, with some companies suggesting widespread market release as early as 2022; samples are already making their way to consumers for market testing. As a product, it has the potential for a tailored nutritional profile. As an industry, it promises a more sustainable and cruelty-free option. However, current production needs to be more efficient and cost competitive for scale-up to even attempt economic comparison with the conventional meat sector, and extensive life cycle assessment is needed to determine its environmental sustainability. This remains a tall order until technology and production costs can approach a more ideal scenario at industry-level scales. UC Davis techno-economic modeling predicts this would require huge reductions in growth media costs from several hundred dollars to just cents per kilogram of cell-based meat, along with vastly improved cellular metabolic efficiency, highly accelerated cell growth, and significantly reduced maturation times (one-third to one-tenth of the current rates). According to the study, this is the only point at which cell-based meat production could foreseeably become economically viable as an alternative commodity to the traditional meat industry.
So, what does that mean for the future of this technology? Until these advances or investments are made, higher value specialty food markets may be the only path forward to low volume, high-cost production. Bring on the cell-based caviar!
Mishi Vachev is a graduate student in the Plant Biology program at UC Davis. She works on finding genes that lead to resistance to fungal diseases in strawberry in Dr. Steve Knapp’s lab. She also is studying genes that control how strawberries respond to daylight length and how that affects fruit production. You can follow her on twitter @MishiVachev.
For more content from the UC Davis science communication group "Science Says", follow us on Twitter @SciSays.
Comments