Stem Cell Therapy: The Future of Healthcare?
In recent years, stem cell therapy has garnered a significant amount of attention. Are stem cell based-therapies for regenerative medicine the answer to an aging and ailing population? First, we should discuss what stem cells are, and why researchers want to use them in medicine. Then how could stem cells be used in the future, and what major step the healthcare industry, as a whole, will need to take to use stem cells for therapeutic solutions on a mass scale.
What are Stem Cells?
Stem cells are undifferentiated cells capable of giving rise to more cells of the same type, and certain other cell types through differentiation. Cells differentiate when they change from one type of cell to another (e.g. a stem cell to a bone cell). Differentiating a stem cell can be achieved through chemical (growth factors), mechanical (applied force such as shear or compression), or genetic (delivery of a gene) stimulation. The full capacity and control of stem cell differentiation is not currently known and is being widely researched.
When people think of stem cells, they often think of embryonic stem cells and the ethical concerns surrounding their acquisition. However, there are stem cells in adult tissues that are found in bone marrow or an umbilical cord. Stem cells can also be acquired by reprogramming skin cells, called induced pluripotent stem cells (iPSCs). My own PhD research focused on facilitating the differentiation of adult stem cells acquired from bone marrow.
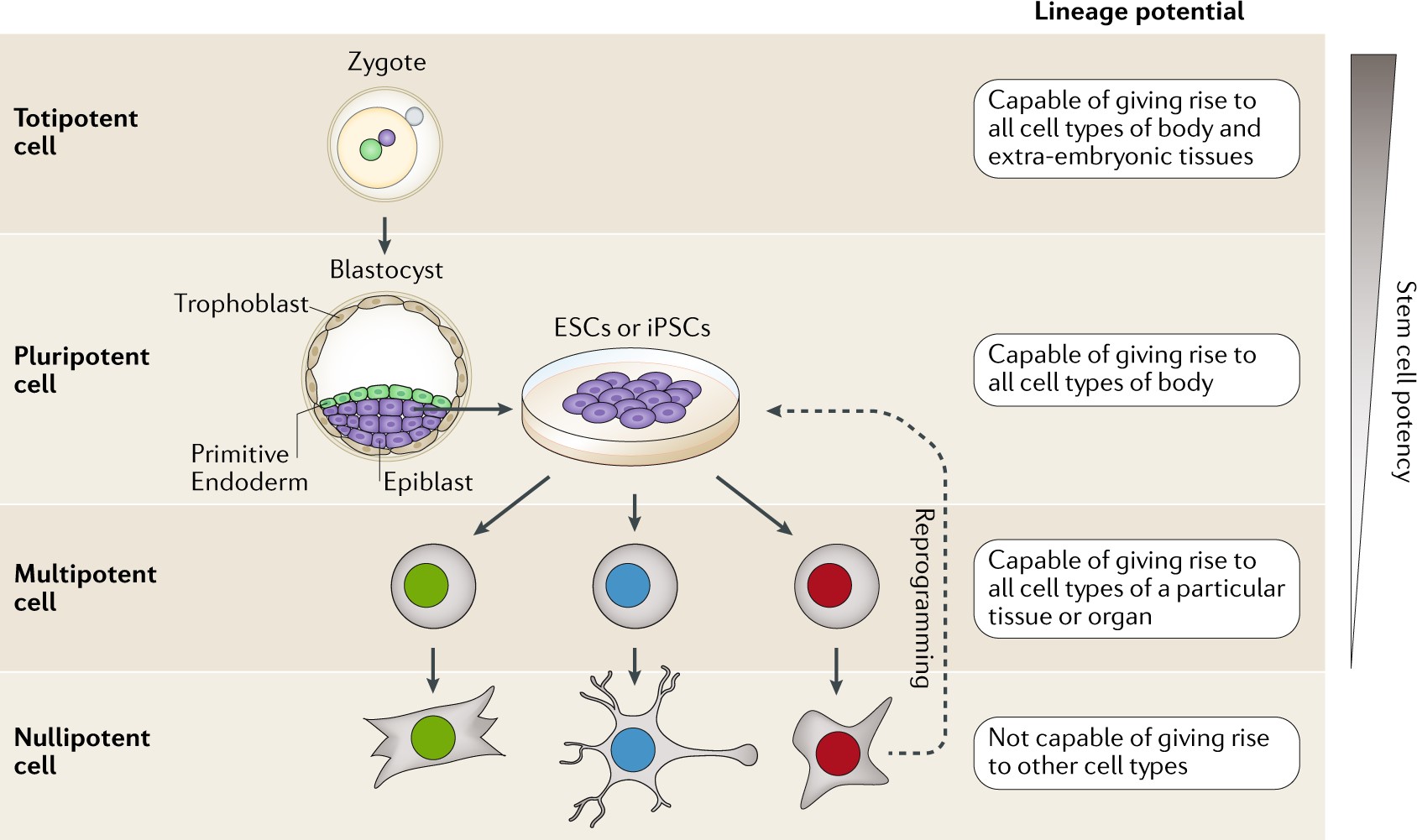
The most important feature of stem cells is their ability to differentiate into other specialized cells. This ability is termed a stem cell’s potency. Thus, stem cells can be totipotent, pluripotent, multipotent, or nullipotent. Totipotent (total potency) cells can become any cell in the human body, including extra-embryonic tissues like the placenta. Pluripotent (several potency) cells can become any cell in the human body. Multipotent (more than one potency) cells can become many different types of cells but only within the same germ layer (or specialized category of cells ie mesoderm). Nullipotent (zero potency) cells cannot become any other type of cell other than itself (e.g., if the cell is a neural cell, it will stay a neural cell). The graphic below also visual describes the lineage potency of stem cells.
Stem Cells in Healthcare
The potency of stem cells is so important because of their potential use in regenerative medicine. For example, osteoarthritis — a disease in which cartilage breaks down or degenerates — is a prevalent problem in older adults. It is incredibly painful and drastically hinders mobility. Obtaining a cartilage transplant or substitute is risky due to implant rejection by an immune system attack, or difficulty and pain resulting from obtaining cartilage from elsewhere in the body. A rejection response by the immune system can render even more joint pain and intensify the osteoarthritis. Doctors aim to limit continued cartilage loss at the expense of managing consequential side effects. However, if researchers could regenerate the lost cartilage with stem cell-based therapies and simultaneously mitigate the risk of rejection, the quality of life for osteoarthritis patients would drastically improve.
With proper control over the stem cell differentiation process, the possible treatments for ailments is innumerable. Researchers facilitating stem cell differentiation strive to work with physicians, nurses, and healthcare staff to bring these therapies from the laboratory bench to the bedside. However, safety is key.
As researchers, we need to support these new treatments by promoting good manufacturing practices (GMPs) in stem cell clinics. To paraphrase Jan Nolta, the director of the Stem Cell Program at the UC Davis School of Medicine and Institute for Regenerative Cures, if a stem cell clinic asks you to pay a large sum of money, it is a big red flag. Typically, these clinics are unregulated by the Food and Drug Administration (FDA) and do not exercise safe practices. A cautionary tale of three patients at a clinic using stem cells therapies with unregulated federal oversight going blind is just one example. However, researchers and medical practitioners who exercise caution and utilize GMPs can overcome these negative perceptions with positive communication about stem cell therapies.
Stem Cells in the Future
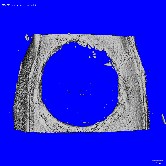
For my PhD research, I worked to facilitate differentiation of multipotent stem cells. With specific tailored factors these stem cells could differentiate in a controlled manner. I worked for years to facilitate their differentiation into bone cells. When I delivered the stem cells to a bone injury, new bone developed successfully. This could influence the development of stem cell-based therapies for bone-related injuries, like non-union fractures. We used micro-CT, a 3D imaging technique utilizing X-rays to see mineralized tissue, to examine the success of new bone development in a bone injury (hole). When no stem cells were provided, no bone formed (left). When stem cells and other required factors were provided, significant bone formation occurred (right).

Bringing Stem Cell Therapies to the Mass Market
Researchers are trying to develop stem cell therapies for the future of medicine with the support, caution, and interest of the general public. Stem cell therapies have tremendous potential to improve and save lives, but must be vetted through clinical trials. Encouragingly, as of December 2019, there are 791 clinical trials underway using human multipotent stem cells. I encourage you to track active clinical trials at the National Institutes of Health website. Use a healthy amount of skepticism and do your own additional research about stem cell therapies.
Takeaway Message
Stem cell-based therapies have a remarkable potential to change the future of medicine. To ensure the safety of those on the cutting edge of regenerative medicine-focused research, the general public must be wary of clinics that exploit peoples’ desperation for a cure. Researchers are working to use stem cells to improve healthcare, but it must be done safely, with some form of oversight.
Jackie Woods is a Science Communication Intern and a PhD candidate in the Biomedical Engineering department. For more content from the UC Davis science communication group "Science Says", follow us on Twitter @SciSays.
Comments